What is a Battery?
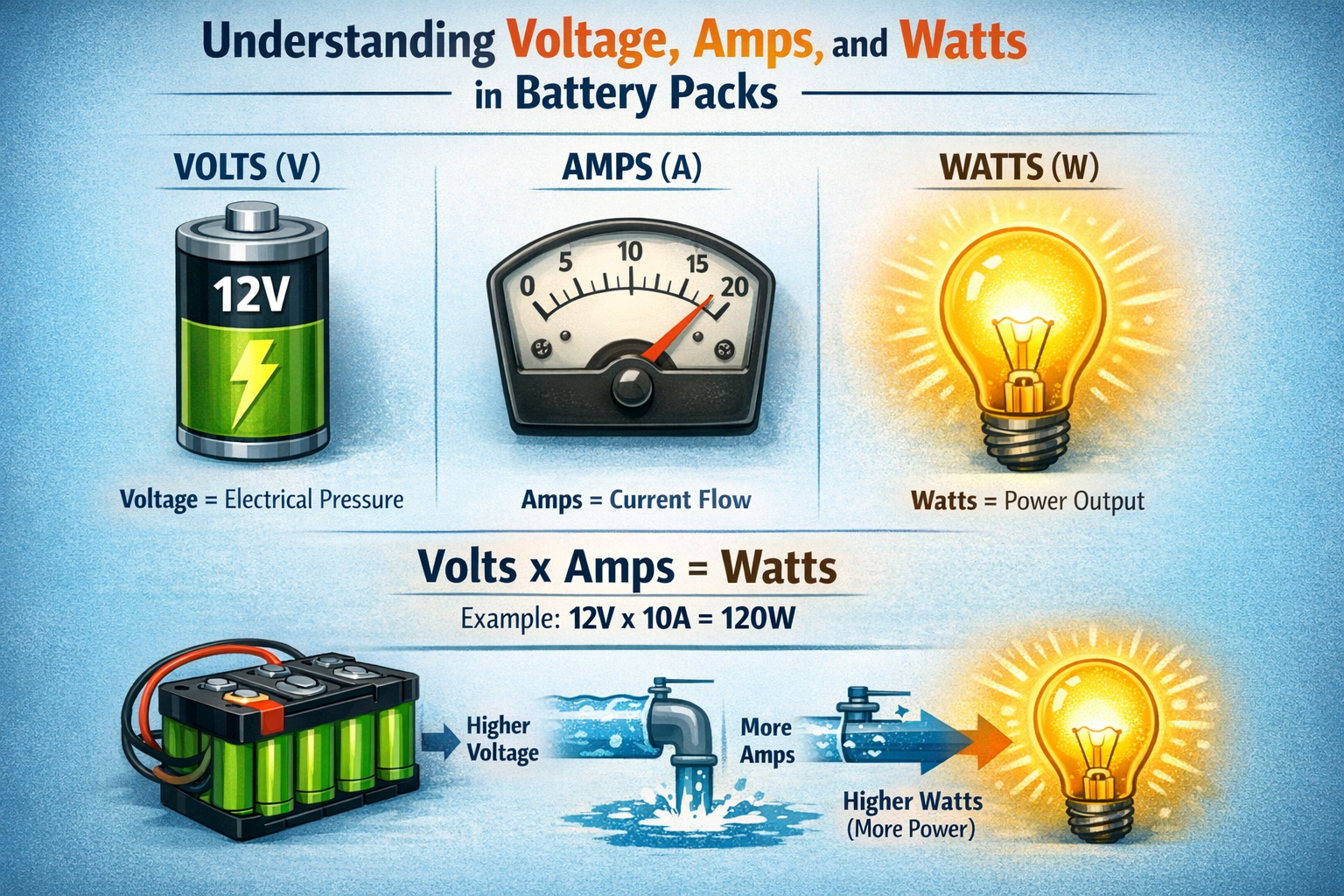
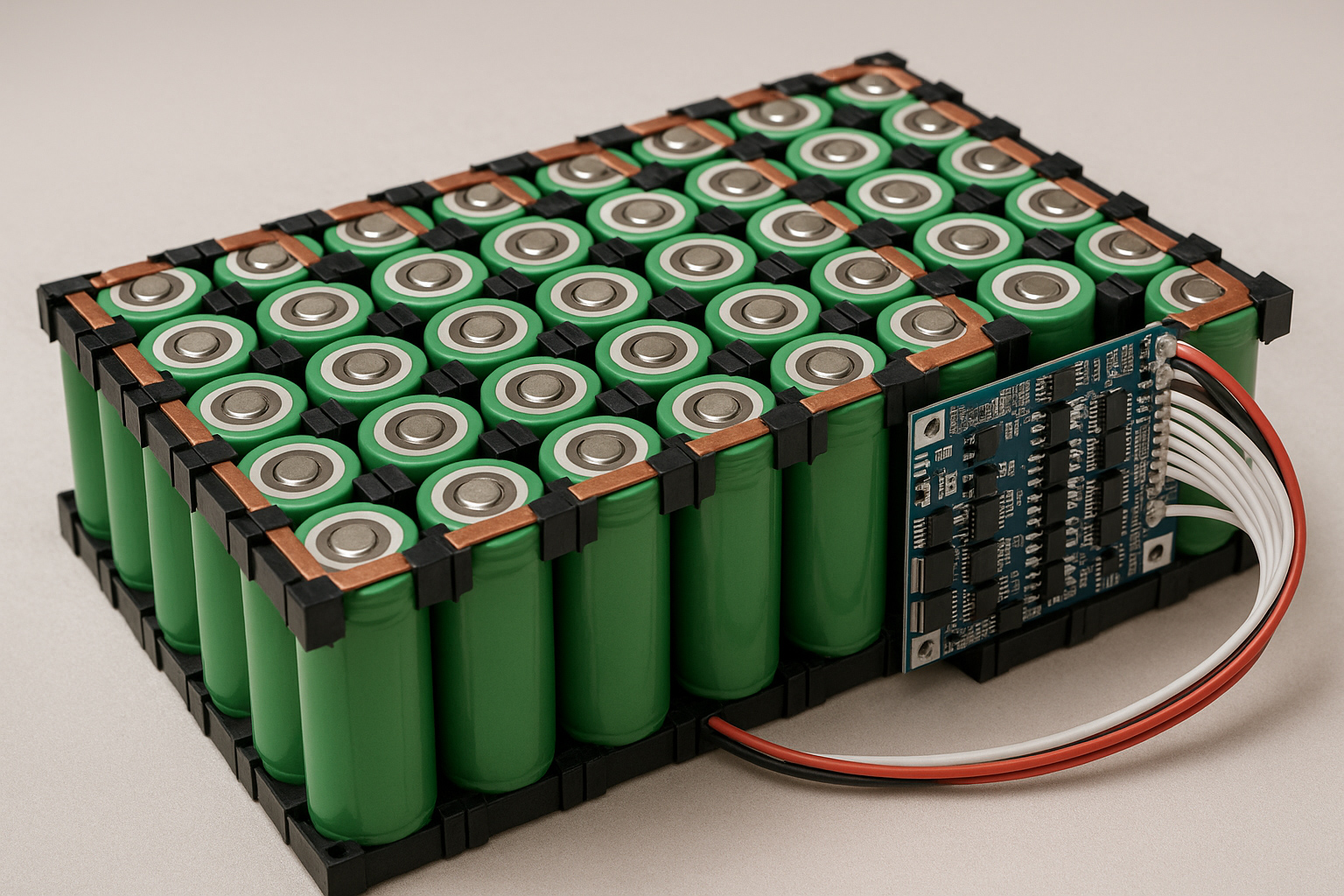
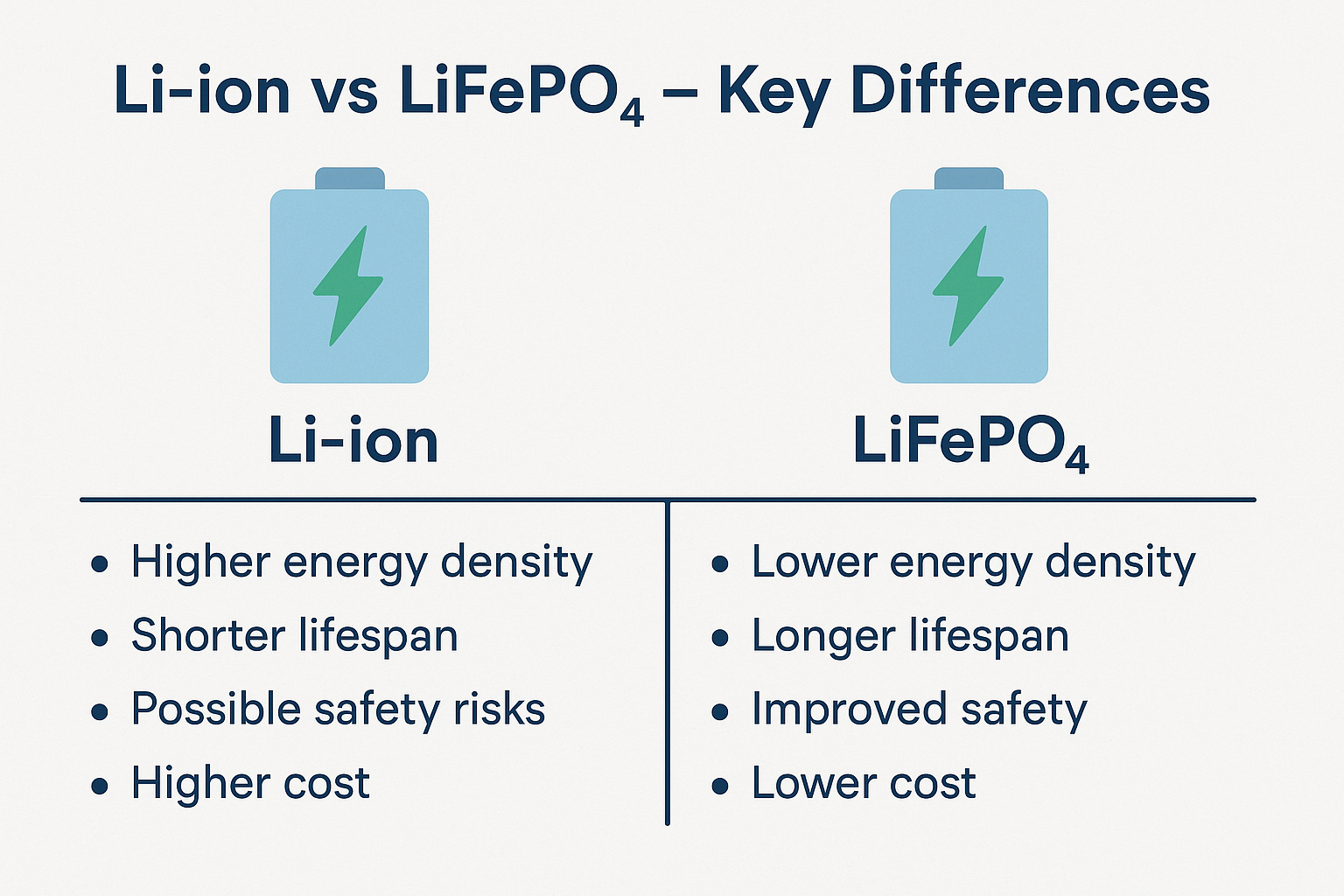
Let's talk batteries. You probably have one within arm's reach right now – maybe two or three. They're the silent partners in our digital lives, the unsung heroes tucked away in gadgets, vehicles, and increasingly, our homes. But what exactly is a battery? It seems like a simple question, but as someone who's spent over 15 years designing, testing, and sometimes cursing these little power packs, I can tell you there's far more beneath the plastic casing than meets the eye. Forget the textbook definitions for a moment; let me pull back the curtain on what makes these electrochemical marvels tick, based on countless hours in the lab and real-world headaches (and triumphs!).It's Not Magic, It's Chemistry (Mostly)At its absolute core, a battery is a self-contained electrochemical device that converts stored chemical energy directly into electrical energy. Sounds sterile, right? Let me translate that into something tangible. Think of it like a tiny, controlled power plant. Instead of burning coal or splitting atoms, it uses carefully chosen chemicals that want to react with each other. My job, fundamentally, is to orchestrate that reaction in a way that gives us useful electricity, safely and reliably, for as long as possible.Anode, Cathode, Electrolyte – The Power TrioEvery battery, whether it's the AA in your TV remote or the massive pack propelling an electric bus, relies on three essential components:The Anode (The Giver): This is where oxidation happens. In simpler terms, it's the electrode that gives up electrons during discharge. Think of it as the energetic kid eager to share their toys (electrons). Common materials? In your alkaline AA, it's typically zinc powder. In your phone's lithium-ion battery, it's often graphite. I've spent countless hours optimizing anode structures – porosity, particle size, coatings – trying to squeeze out every last milliamp-hour without compromising safety. Trust me, dendrites (nasty little metallic growths) are the enemy!The Cathode (The Taker): This is where reduction happens. It accepts the electrons flowing through your circuit. It's the calmer kid who happily receives the toys. Materials vary wildly: manganese dioxide in alkalines, lithium cobalt oxide (LCO) in many older phone batteries, lithium iron phosphate (LFP) gaining popularity for its stability, or nickel-manganese-cobalt (NMC) blends common in EVs. Choosing the right cathode chemistry is a constant balancing act between energy density, power output, cost, cycle life, and safety – a puzzle I grapple with daily.The Electrolyte (The Traffic Cop): This is the crucial, often overlooked, mediator. It's the medium – usually a liquid, gel, or increasingly, a solid – that allows ions (electrically charged atoms/molecules) to shuttle back and forth between the anode and cathode internally, balancing the flow of electrons externally through your device. Without the electrolyte completing the internal circuit, nothing happens. It's the highway system inside the battery. I've seen batteries fail spectacularly because the electrolyte degraded, dried out, or became contaminated. Getting this chemistry right is paramount.The Dance of Discharge and ChargeHere's where the magic (okay, science) happens:Discharge (Using the Battery): When you turn on your device, you complete the external circuit. Electrons, desperate to flow from the anode (high energy state) to the cathode (lower energy state), rush out through your device, powering it. Simultaneously, to maintain electrical neutrality inside the battery, positively charged ions (like Li+ in lithium-ion) travel through the electrolyte from the anode to the cathode. The chemical reaction proceeds, releasing energy as electricity. The battery discharges.Charge (Refueling the Battery): Plugging in your charger reverses the process. You force electrons back into the anode (against their natural flow) using external power. This drives the ions back through the electrolyte from the cathode to the anode. The chemical reaction is reversed, storing energy back in the chemical bonds. The battery charges.The Real-World NuancesUnderstanding the core trio and the electrochemical dance is essential, but it's only the start. What makes battery engineering fascinating (and sometimes frustrating) are the complexities layered on top:The Separator: Imagine a very porous membrane porous membrane sitting physically between the anode and cathode. Its job? To prevent them from touching and causing a direct short circuit (which leads to heat, fire, bad news), while still allowing ions to pass freely through its pores. I've analyzed separator failures under microscopes – a tiny puncture can be catastrophic. Material science here is critical.The Case & Terminals: It's not just a plastic box! The case must contain potentially reactive materials, withstand internal pressure changes, resist environmental factors, and provide electrical insulation. Terminals need low resistance connections. I've designed custom cases for harsh environments – think desert heat or Arctic cold – where material choice becomes life-or-death for the battery.Battery Management System (BMS): Especially crucial for multi-cell packs (like in laptops, EVs, power tools). This is the battery's brain. It monitors voltage, current, and temperature of individual cells or groups. It balances cells to ensure they charge/discharge evenly (preventing weak cells from being overstressed). It enforces safety limits – shutting down if things get too hot, overcharged, or short-circuited. A poorly designed BMS is a ticking time bomb. I've debugged BMS firmware late into the night more times than I care to admit!Common Types & Where They Shine (Or Fizzle)Not all batteries are created equal. Choosing the right one depends entirely on the application. Here's my practical take on the major players:Alkaline (Zn/MnO2): The workhorse. Cheap, decent shelf life, safe. Powering your remotes, clocks, basic toys. Pros: Inexpensive, readily available, decent energy density for cost. Cons: Poor performance in high-drain devices (digital cameras drain them fast!), voltage drops steadily during discharge, not rechargeable. My Tip: Don trying trying to recharge standard alkalines – it's ineffective and potentially dangerous. For low-drain devices, they're still hard to beat for cost.Lithium-Ion (Li-ion) & Variants (Li-Poly, LFP, NMC, etc.): The undisputed king of portable electronics and EVs. Pros: Very high energy density (lots of power in a small space), high voltage per cell (~3.7V nominal), relatively low self-discharge, rechargeable hundreds/thousands of times. Cons: More expensive, requires sophisticated BMS for safety (thermal runaway risk if damaged/abused), performance degrades with age and temperature extremes. My Experience: Handling damaged Li-ion cells requires extreme caution. I've seen thermal events triggered by seemingly minor punctures. Treat them with respect! Advice: Avoid deep discharges and extreme heat/cold for longest life. Partial charges are better than full 0-100% cycles.Nickel-Metal Hydride (NiMH): The reliable rechargeable. Pros: Good energy density (better than NiCd), no "memory effect" (mostly a NiCd issue), safer chemistry than Li-ion, good for high-drain devices. Cons: Higher self-discharge than Li-ion (they lose charge sitting on the shelf), lower voltage per cell (~1.2V), performance drops in cold temps. My Go-To: Still fantastic for high-drain rechargeables like cordless power tools (where Li-ion dominates now but NiMH persists), high-quality AA/AAA rechargeables, some hybrid vehicles. Buy low-self-discharge (LSD) NiMH brands if they'll sit unused.Lead-Acid (Flooded, AGM, Gel): The old faithful. Pros: Inexpensive, robust, tolerant of abuse, high surge current (great for starting engines), recyclable. Cons: Very heavy, low energy density, slow charging, contains toxic lead and sulfuric acid, limited cycle life if deeply discharged. My Reality Check: Still essential for car starting batteries (though Li-ion is creeping in), uninterruptible power supplies (UPS), golf carts, marine applications. AGM (Absorbent Glass Mat) versions are sealed and spill-proof, making them popular upgrades. Tip: For deep-cycle applications (like RVs), avoid discharging below 50% Depth of Discharge (DoD) for maximum lifespan.Living With Batteries: Hard-Won WisdomBeyond the specs, here's practical advice forged from blown-up prototypes, customer complaints, and successful deployments:Temperature is the Silent Killer: Heat accelerates all degradation mechanisms inside a battery. Cold drastically reduces available capacity and power (especially for Li-ion and NiMH). Store and use batteries within their specified temp ranges whenever possible. That phone left on the dashboard in summer? Murdering its battery.Depth of Discharge (DoD) Matters: Draining a battery completely stresses it far more than using only part of its capacity. Shallower discharges generally mean longer overall lifespan. Think of it like exercise – moderate is sustainable, extreme takes a toll.Charging Wisely: Use the charger designed for your battery type! Overcharging is destructive. Trickle charging lead-acid is fine; doing that to Li-ion degrades it. Fast charging generates heat and stress – use it when needed, not as a default. That "overnight charge" habit? Not ideal for Li-ion longevity.Storage: For long-term storage (months), Li-ion likes to be around 40-60% charge in a cool place. NiMH should be stored charged. Lead-acid needs periodic topping charges. Storing a Li-ion battery fully discharged can permanently damage it.Safety First: Never physically damage a battery, especially Li-ion. Don't expose them to fire or extreme heat. Recycle responsibly – heavy metals and reactive chemicals don't belong in landfills. If a battery is swollen, leaking, or excessively hot, stop using it immediately and dispose of it safely.Where My Passion LiesThe battery world is exploding (figuratively, hopefully!). We're pushing the boundaries of energy density with silicon anodes and advanced cathodes. Solid-state batteries promise even higher density and significantly improved safety by replacing flammable liquid electrolytes with solid ones – a holy grail I'm deeply involved in researching. We're exploring sodium-ion as a potentially cheaper, more sustainable alternative to lithium. Recycling technologies are advancing rapidly to recover valuable materials. The push for grid-scale storage to support renewable energy is driving innovation in flow batteries and other large-scale chemistries.So, what is a battery? It's more than just a power source. It's a meticulously engineered electrochemical system, a triumph of materials science and chemistry. It's the quiet enabler of our mobile lives, the key to unlocking renewable energy, and a field brimming with exciting challenges and innovations. From the humble AA to the massive packs powering the future, understanding what happens inside that unassuming case empowers us to use them smarter, safer, and longer. Next time you pick one up, remember the intricate dance of electrons and ions happening within – it's a tiny piece of engineering brilliance you hold in your hand. And trust me, we engineers are working tirelessly to make that dance even better.
10 Aug 2025
Read More
 Battery Basics
Battery Basics